PatenSee’s Contactless Imaging Device Shows Promise in Timely and Accurately Monitoring Dialysis Patients
Clinical results revealed today in a presentation at the WCN22
Or Yehuda, Israel, February 24, 2022 — The interim results of an ongoing clinical study of PatenSee’s contactless imaging device for the early detection of vascular access stenosis for dialysis patients were shared today in a poster presentation. The presentation, titled First Clinical Experience with Non-Invasive, Contactless, Optical Surveillance of Vascular Access, was delivered by the study’s principal investigator, Professor Benaya Rozen-Zvi, MD at the 2022 World Congress of Nephrology Meeting in Indonesia.
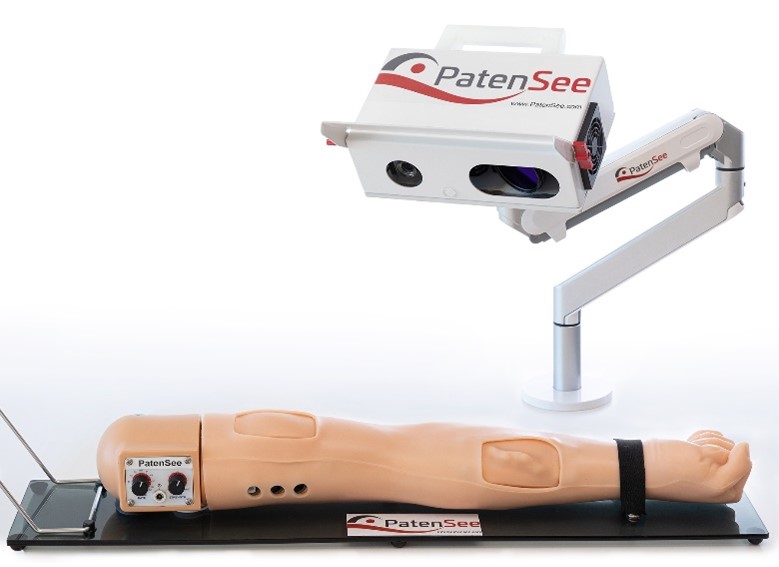
Caption: PatenSee’s vascular access stenosis detection device
The vascular access fistula (AVF) connects the patient, usually from their arm, to the dialysis machine. This makes it critically important to protect the AVF from clogging. A blocked access can cause various complications including loss of the AVF and death. The goal is to detect initial clogging before it leads to an unwanted patient outcome, but ongoing physical monitoring by the medical team is a major challenge. PatentSee’s imaging technology is designed to perform quick, high-quality monitoring without any physical contact with the patient, thus reducing risk of infection, especially important in pandemic times.
“Avoiding AVF stenosis has never been more important. Dialysis treatment centers are very busy, physical examinations take time and require touching an already sensitive area on the patient, plus there is the risk of infection from contact, especially in our new COVID reality. The development of a contactless system that can nearly instantly provide accurate information of an AVF’s status couldn’t have come sooner and the clinical results thus far demonstrate that PatenSee’s technology has strong potential to ease the burden of AVF monitoring,” said PatenSee CEO Dr. Gal Goshen.
Thirty-six dialysis patients participated in more than 600 sessions collectively at Rabin Medical Center in Israel to test the efficacy and timeliness of PatenSee’s imaging device.
Imaging took approximately two minutes and was performed during the waiting time prior to the dialysis sessions. A comparison to a doppler ultrasound done by a technician blinded to the results of PatenSee’s device demonstrated good correlation suggesting that this rapid, contactless, machine learning, imaging device could serve as an effective tool for automated AVF surveillance.
“Surveillance of the AVF by inspection and clinical examination is a challenging task and many times is not performed adequately. As a result, early stenosis is frequently not detected in a timely manner, which leads to access loss and the need for potentially harmful temporary catheters. The interim results of the current clinical study show that PatenSee’s imaging device has the potential to fix this problem. More research is required to evaluate the system as a predictor of AVF stenosis” said Professor Rozen-Zvi.
About PatenSee
PatenSee, a portfolio company of the MEDX Xelerator, is a clinical-stage medical device company developing a contact-free imaging surveillance system for the early detection of vascular stenosis in hemodialysis patients. Using advanced, multi-modal imaging technologies and machine learning, PatenSee’s device aims to alert regarding stenosis risk at the earliest stage, enabling timely interventions to protect the patient’s hemodialysis lifeline, improve quality of care and prolong life expectancy in both the clinic and the home dialysis setting. The dialysis market is expected to exceed $100B by 2024. PatenSee is currently raising a series A to fund the next stages of its clinical program towards the commercial launch of the product. For more information, please visit www.patensee.com.
Follow PatenSee on LinkedIn.
Press Contacts:
Marjie Hadad
General Manager
Must Have Communications
917-790-1178
011-972-54-536-5220 (WhatsApp)
Back